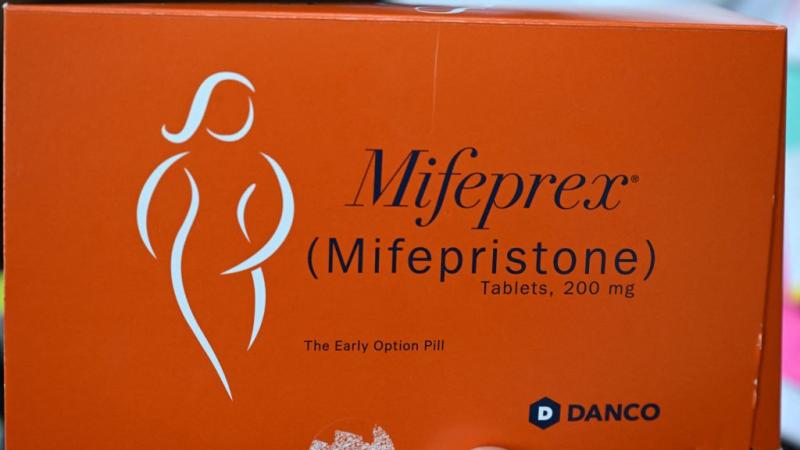
Senators urge HHS, FDA to reevaluate approval of a new generic version of mifepristone
The FDA sent a letter approving the drug to its manufacturer, Evita Solutions LLC, last week stating that it has reviewed the drug and found that it meets the requirement for approval under the Federal Food, Drug, and Cosmetic Act.
A group of senators on Thursday urged Health Secretary Robert F. Kennedy Jr., and Food and Drug Administration Commissioner Marty Makary to reevaluate the approval of a new generic version of the chemical abortion drug mifepristone.
The FDA sent a letter late last month to Evita Solutions LLC saying the agency had reviewed the drug and found that it meets the requirement for approval.
South Carolina Republican Sen. Lindsey Graham led the group of senators in signing and sending the letter, first shared with Just The News, which generally supported the leaders but encouraged them to take decisive action on reevaluating the drug.
"Under your leadership, we have seen a strong commitment to reevaluating the policies that affect the most vulnerable among us – the unborn," the senators wrote. "We applaud your acknowledgment of the concerns surrounding the FDA’s approval and regulation of the abortion pill regimen – mifepristone and misoprostol – and your commitment to following the science to ensure the safety and well-being of women and unborn children alike. But the work is far from over.
"The 'abortion-on-demand' culture enabled by the Biden-Harris administration’s removal of critical safeguards on the only FDA-approved abortion regimen is currently the biggest threat to unborn life in America today," the group continued. "Under current FDA regulations, these drugs can be obtained via mail order without meaningful consultation with a medical professional and without any confirmation of who is purchasing them or for what purpose."
The senators urged the agency leaders to suspend approvals of other generic forms of the abortion drugs until they conduct a safety review, ensure that all generic forms of mifepristone are included in the review, reinstate in-person dispensing requirements for mifepristone and its generic forms, and pull FDA guidance that allows pharmacies to distribute the drug.
“If the federal government allows the abortion pill to be sent by mail, it would wipe out all state laws that protect the unborn, particularly states that restrict abortion at twelve weeks and under," Graham told Just The News. "If you believe that protecting the unborn is a state issue, you cannot have the federal government undercutting that position by allowing the abortion pill to be sent through the mail.
"It is important for the Trump Administration to return to its 2017 policies, requiring a doctor consult before the drug can be prescribed because of the dangers it presents," he continued. "One of the most important issues in the history of the pro-life movement is the chemical abortion pill and how it’s administered. We must get this right."
The letter was signed by a total of 51 Republican senators, including Senate Majority Leader John Thune, Senate Judiciary Chairman Chuck Grassley and Indiana Sen. Jim Banks.
The FDA approval of the drug comes after HHS said last month that it would conduct a study on the real-world harms of chemical abortion drugs.
Misty Severi is a news reporter for Just The News. You can follow her on X for more coverage.